Medicines Access
Our tailored medicines access solutions are where our clients really understand the value of working with us.
Overview
BAP Medicines Access
As pharmaceutical and biotechnology companies face increasingly complex hurdles to bring their medicines to the market and secure national funding, ensuring continued access to sought after medicinal products for patients is becoming ever more important.
There is more pressure than ever for companies to maximise the potential of their asset throughout the product lifecycle.
Although challenging, providing access to unlicensed, pre-approved medicines can form a critical component of an access strategy.
BAP Pharma can work with you to provide bespoke medicines access programs to ensure patients can get the treatment they need, whether it is pre-license, post-MA or as internal resource is reduced, support with withdrawal management.
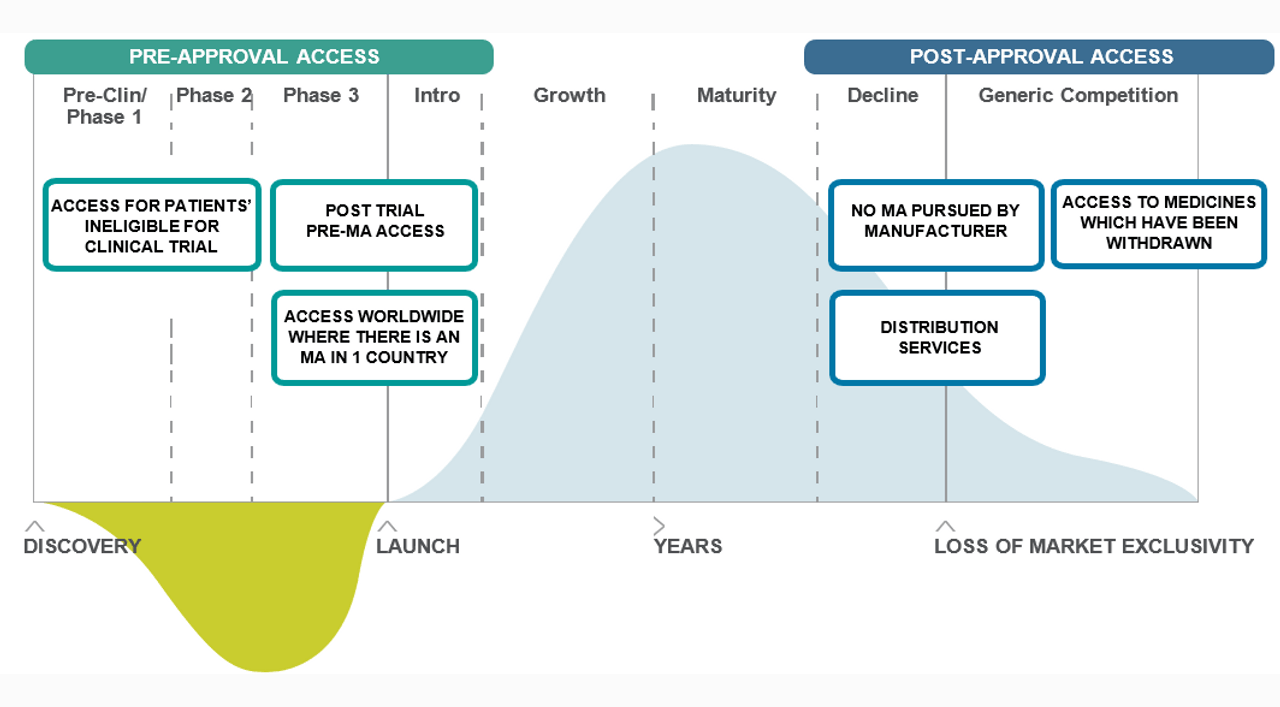

Pre-Approval Access
- During clinical trials, patients may not be able to access a trial medicine either because they do not meet the inclusion criteria or do not have access to the trial.
- Post clinical trials, a trial medicine may never make it into the market of certain countries, or a patient may need to continue treatment until it does.
Post-Approval Access
- After clinical trials, a medicine may not be registered in the market or in certain countries for many years, or ever. BAP Pharma can help with the supply to these countries.
- A medicine, or a license, may also be withdrawn from a market.

BAP Pharma Added Value
BAP Pharma provide Medicines Access solutions for biopharmaceutical manufacturers in consulting, advising, designing, and delivering medicines access programs to ensure patients can get access to medicines with or without a marketing authorisation.
- Consulting
We provide market intel and strategic input to enable wider access to medicines. - Development of Bespoke Access Solutions
We partner with customers to discuss how we can help them broaden access to medicines so patients in need can benefit from treatments. - Regulatory and Quality Expertise
Our in-house team have extensive knowledge to ensure access to your medicine is compliant and in line with country specific regulations. A key strength is our access to documentation, translations & local language labels to ensure ease of use at point of administration to the patient - Safe Storage and Global Distribution
We can support distribution of approved and unapproved medicines across the globe, with state-of-the-art facilities and expertise in customs clearance.
